Our planet is protected by an essential shield in the sky - the ozone layer. Situated in the stratosphere, this layer contains a high concentration of ozone (O3) molecules, safeguarding life on Earth from harmful ultraviolet (UV) radiation. The ozone layer plays a crucial role in preventing skin cancer, cataracts, and other health issues in humans, while also preserving ecosystems and wildlife. In this blog, we will explore the history of ozone layer depletion, the scientific processes behind ozone formation and depletion, the role of the Dobson spectrophotometer in measuring ozone depletion, the success of the Montreal Protocol in protecting the ozone layer, and the challenges and progress towards the ozone layer's recovery.
History of ozone layer depletion:
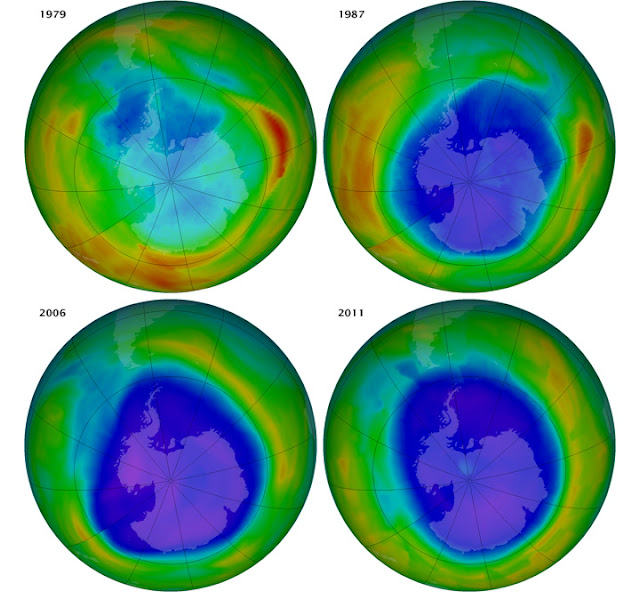
In the 1970s, scientists made a grave discovery - human-made chemicals, particularly chlorofluorocarbons (CFCs) and halons, were causing significant damage to the ozone layer. Widely used in refrigeration, air conditioning, and aerosol propellants, these chemicals posed a severe threat to the Earth's protective layer. The alarming revelation spurred early efforts to understand the causes and effects of ozone depletion.
The Chapman cycle:
At the heart of the ozone layer's formation and destruction lies the Chapman cycle. Solar UV radiation breaks down oxygen molecules (O2) into individual oxygen atoms (O). These atoms can then combine with other oxygen molecules to form ozone (O3). Conversely, ozone can be destroyed when it absorbs UV radiation, breaking down into oxygen atoms and molecules again. This continuous cycle regulates the concentration of ozone in the stratosphere.
The Dobson spectrophotometer:
To measure the percentage of ozone depletion, scientists developed the Dobson spectrophotometer. This groundbreaking device analyzes the amount of UV radiation absorbed during sunrise and sunset. By calculating the ozone layer's thickness in Dobson Units (DU), which represents the number of molecules required for a layer of pure ozone at standard temperature and pressure, scientists can monitor ozone depletion levels.
The Montreal Protocol:
In 1987, the world witnessed unprecedented international cooperation through the adoption of the Montreal Protocol. This landmark treaty aimed to phase out the production and consumption of ozone-depleting substances (ODS), including CFCs and halons. The treaty's success has led to a significant reduction in ozone depletion, showing the potential when nations unite to protect the environment.
The future of the ozone layer - Progress and challenges:
Since the implementation of the Montreal Protocol, there have been promising signs of recovery. According to the United Nations Environment Programme (UNEP), the ozone layer is on track to recover within four decades. A significant report released in January 2023 by the UNEP's Ozone Secretariat revealed that the ozone layer over the Antarctic has been recovering at a rate of 1-3% per decade since 2000. This positive trend indicates that the ozone layer is expected to return to its pre-1980 levels by the mid-2060s.
However, challenges remain on the horizon. One such concern involves the introduction of hydrofluorocarbons (HFCs) as a replacement for CFCs. Although HFCs do not deplete the ozone layer, they are potent greenhouse gases, contributing to global warming. As we continue to protect the ozone layer, we must also address the impact of alternative substances on climate change.
The ozone layer depletion crisis taught us vital lessons about the impact of human actions on the environment. Through international collaboration and prompt action, the Montreal Protocol showcased the collective power of protecting our planet. As we forge ahead, the success of the treaty serves as a reminder of our ability to overcome environmental challenges through cooperation and innovation. Let us remain vigilant in safeguarding our skies and preserving the ozone layer for generations to come. Together, we can ensure a brighter and healthier future for our planet.


Comments